Silicone rubber is universally regarded as the best-in-class elastomer, especially for extreme environments. In addition, it is one of the most permeable elastomers. Here, we present a small review of the types of silicones and explain how the curing and processing are different.
 CHARACTERIZATION OF SILICONE RUBBER
CHARACTERIZATION OF SILICONE RUBBER
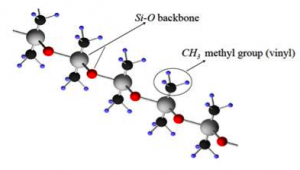
Silicone rubber is a thermoset with a backbone of alternating silicone and oxygen atoms with methyl or vinyl side groups. There are two types of silicone rubber: solid and liquid. The basic structure of the two types of silicone rubber is the same, but curing and processing are radically different.
TYPES OF SILICONES AND THEIR PROCESSING
High Consistency Rubber (HCR) is produced in large batches; the components are mixed at high temperatures and a peroxide catalyst is added. During the reaction, the vinyl double bond is reactive and the peroxide group produces an oxygen-free radical. A reactive-free radical is formed on the vinyl group and the free radical attaches itself to another polymer, forming a bridge. The free radical chain reaction then continues. Only some cross-linking occurs because the process is interrupted before vulcanization is complete to form sheets and the materials are stored. After that, the sheets are mixed with rollers and processed by transfer molding. The final vulcanization occurs in an oven (post-vulcanization).
Liquid Silicone Rubber (LSR) is a two-component system. Component A contains a platinum catalyst and Component B contains methylhydrogensiloxane as a d cross-linker and an alcohol inhibitor.
The reaction starts with the reactive vinyl double bond; the platinum center has one free coordination site. The interaction with the platinum center activates the double bond and the vinyl group cross-links by transforming the double bond, creating a single bond to a polymer chain (in this case, to a cross-linker molecule containing Si-H groups). The catalyst becomes free and is again available for further cross-linking. During the processing, the mixing is precise and no posturing is required. All curing occurs in the mold and the parts obtained have good repeatability.
During the vulcanization, the long chains of the elastomer chemically cross-link.
Each cross-link releases a quantum of energy, making it an exothermic reaction. During this process, the catalyst establishes bonds between the long chains, creating a three dimensional matrix. This network greatly improves the mechanical properties of the rubber. It is important to understand how silicone rubber vulcanizes in order to optimize the processing conditions and to choose the correct material for each application. To obtain that, it is first necessary to characterize the silicone to determine the thermal and rheological properties in addition to the curing reaction kinetics, a main consideration in the modeling of the curing process.